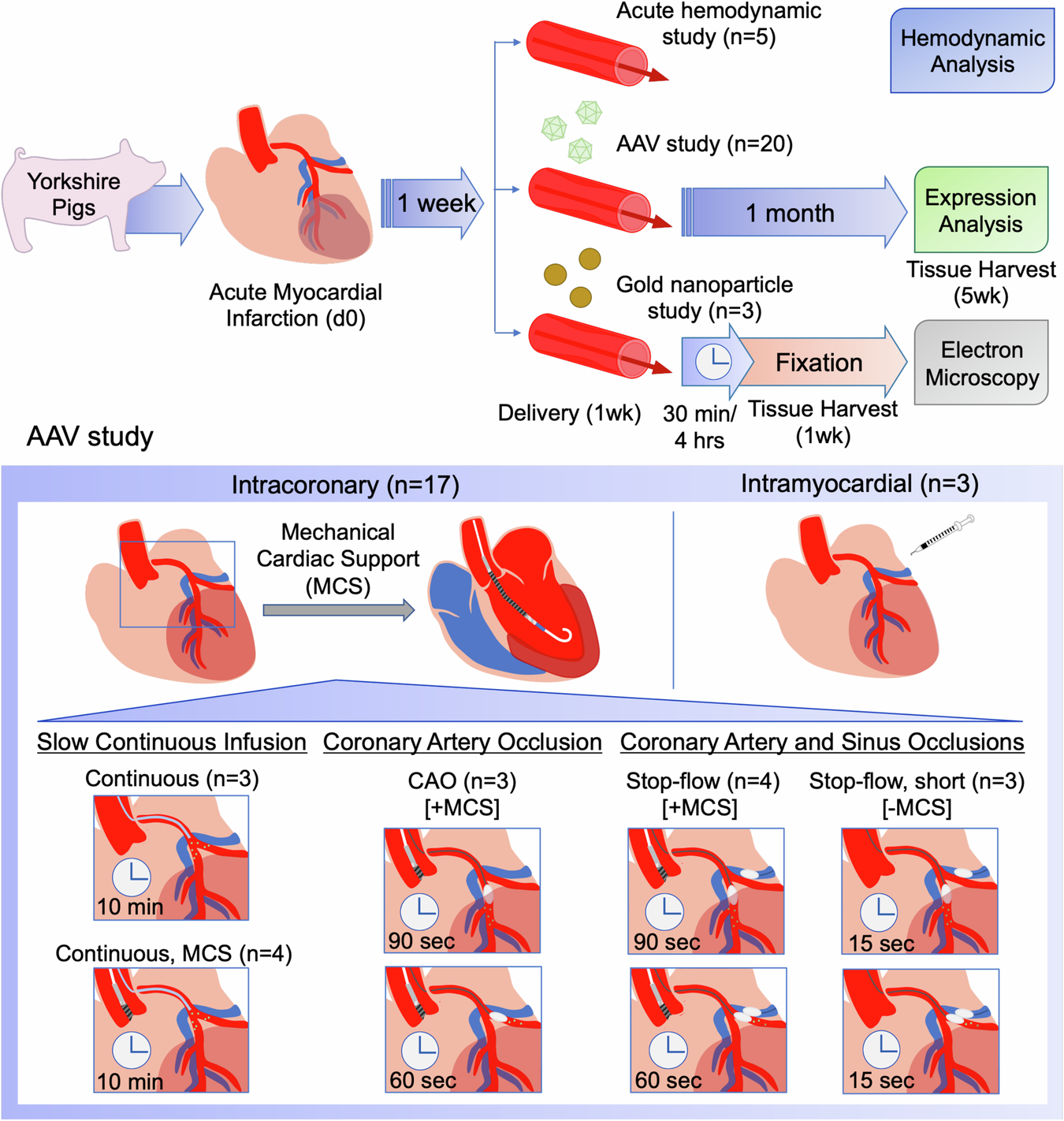
Advancements in Gene Therapy: A Closer Look at Vascular Endothelial Cell Transduction
The field of gene therapy has seen a remarkable surge in innovative approaches over the past few decades. In an era when precision medicine and biotechnology are taking center stage, recent studies have focused on refining adeno-associated virus (AAV) variants to improve the delivery of genes into human vascular endothelial cells. These specialized cells, which line the interior of blood vessels, play a key role in maintaining cardiovascular health. Today, we take a closer look at the latest research, discuss its implications, and offer a perspective on how these modified viral vectors are set to revolutionize treatments for a host of cardiovascular and related conditions.
Overcoming the Tricky Parts of Viral Vector Engineering
The science behind refining AAV variants is both fascinating and full of tricky parts. Researchers are tasked with the challenge of modifying the viral shell – the protein coat – in such a way that it can efficiently interact with and penetrate human vascular endothelial cells. There are several tangled issues involved when working with these vectors, including the need to enhance transduction efficiency without compromising safety.
Many in the field have recognized that a successful gene therapy vector must overcome the following challenges:
- Target Specificity: The vector should ideally identify and attach solely to the intended cells.
- Immune Evasion: It must avoid rapid clearance by the immune system, which is often a nerve-racking concern for scientists.
- Efficient Gene Delivery: The virus must be able to carry therapeutic genes across the cell membrane and into the nucleus with minimal obstruction from the body’s natural defenses.
By tackling these confusing bits, researchers have designed engineered AAV variants that show promise in delivering genetic material more precisely and safely. Such modifications aim to clear away the twists and turns of traditional gene delivery methods, leading to a smoother, more consistent therapeutic effect.
Digging Into the Clinical Significance of Modified AAV Variants
Novel research efforts led by experts in the field have brought forward a study published in the journal Gene Therapy. Here, the investigators focused on tweaking the AAV variants to boost their ability to transduce human vascular endothelial cells. The results suggest that these modified viral vectors could be a game-changer in treatments, particularly for cardiovascular diseases, where the modulation of endothelial cell function is essential.
This study is not just about enhancing the delivery mechanism, but it also provides a blueprint on how to deal with several off-putting obstacles that have kept gene therapy from reaching its full potential in vascular medicine. Improved vector performance could lead to:
- More predictable therapeutic outcomes due to efficient gene expression.
- Reduced risk of immune reactions, thereby making the therapy safer.
- Potential applications in treating diseases linked with endothelial dysfunction, such as atherosclerosis or ischemic conditions.
The clinical implications of these advancements are enormously exciting. With better vector designs, clinicians may soon have more effective tools in their arsenal against life-threatening conditions related to blood vessel health. In this light, the findings of the study serve as an early indicator of a paradigm shift that could eventually translate into improved patient outcomes and quality of life.
Diving Into the Science Behind the Modifications
The process of modifying AAV vectors involves a series of intricate steps where researchers poke around the virus’s protein structure to identify key sites for alteration. These subtle parts of the viral coat are essential in determining how well the virus can bind to specific cell types and deliver its genetic payload.
Some of the critical areas that scientists have targeted include:
- Receptor Binding Domains: Small adjustments in these regions can help the vectors latch on more strongly to the receptors on endothelial cells.
- Capsid Stability: Enhancing the resilience of the viral capsid enables the vector to survive longer in the body, giving it a better chance to achieve successful gene transfer.
- Surface Charge Modulations: Tweaking the electrostatic properties of the viral surface can lower the chances of immune system interference.
These fine points of vector design require a detailed understanding of both the hidden complexities of viral structure and the minute differences that distinguish one cell type from another. By manipulating such little twists in the regimented structure of AAV, researchers can figure a path through what has traditionally been a nerve-racking and overwhelming process of gene delivery optimization.
Exploring Alternative Perspectives on Gene Delivery Challenges
While the progress in vector engineering is promising, it is important to recognize that the journey is still full of problematic, complicated pieces. Innovations in other areas of biotechnology, such as alternative gene delivery mechanisms and non-viral methods, offer a broader perspective on how the hurdles of gene therapy might be overcome.
There are several alternative methods currently under investigation, including:
- Lipid Nanoparticles: Small spherical carriers that encapsulate DNA or RNA, offering a virus-free strategy.
- Polymeric Carriers: Biodegradable polymers that can ferry genetic material with reduced immune responses.
- Physical Methods: Techniques like electroporation or ultrasound-mediated delivery that physically force genetic material into cells.
Each of these methods has its own set of tangled issues – from ensuring adequate gene expression to managing any off-target effects. However, compared to these approaches, AAV-based vectors provide the benefit of high transduction efficiency and long-term gene expression. As such, the improvements in modified AAV variants mark a significant milestone for the gene therapy community.
Assessing the Benefits for Cardiovascular Applications
Cardiovascular diseases remain a leading cause of mortality worldwide. The burden of these conditions is further compounded by the fact that traditional treatments often only manage symptoms rather than address underlying causes. Enhanced gene delivery using modified AAVs marks a step toward treating the root of many of these disorders.
Some key cardiovascular implications include:
- Improved Angiogenesis: Gene therapies that promote new blood vessel formation can help restore blood flow to damaged areas of the heart.
- Stabilization of Atherosclerotic Plaques: By modulating gene expression in endothelial cells, it may be possible to reduce plaque instability that often leads to heart attacks.
- Reduction in Inflammation: Targeting inflammatory signaling in vascular cells can minimize tissue damage and reduce complications arising from chronic inflammation.
Implementing these advanced gene delivery methods into clinical practice may pave the way for novel therapies that not only delay the progression of cardiovascular diseases but also offer hope for regeneration and repair of diseased tissues. This is particularly crucial, as the traditional methods of managing conditions like heart failure or coronary artery disease often fall short of providing a lasting solution.
The Role of Precision Medicine in Gene Therapy
Precision medicine is at the heart of modern healthcare. As treatments become increasingly tailored to individual patients, the ability to deliver genes selectively to diseased tissues is a super important part of that personalized approach. The enhanced AAV variant research is a prime example of how targeting the specific needs of endothelial cells can lead to more effective interventions.
In this context, the use of modified viral vectors aligns with the goals of precision medicine to:
- Personalize Therapeutics: Each patient’s genetic makeup is different. Custom-engineered vectors can be designed to address unique biological profiles.
- Minimize Side Effects: Improved targeting reduces off-target gene expression, which in turn decreases potential side effects and increases overall treatment safety.
- Optimize Dosage: High-efficiency gene delivery means that lower doses may be required, reducing the possibility of immune reactions and toxicity.
This tailored approach helps bridge the gap between laboratory research and patient care, serving as a beacon of hope for many who suffer from conditions that have thus far been categorized by little distinctions in treatment efficacy. As the modified AAV vectors continue to evolve, they promise to become an integral part of a truly personalized therapy regimen.
Practical Considerations for Implementing Modified AAV Therapies
Despite the inspiring advancements, several practical considerations come into play when it comes time to translate these findings into clinical settings. The journey from bench to bedside is loaded with challenges that require careful planning and thorough testing.
Key practical aspects include:
- Large-Scale Production: Engineering and manufacturing sufficient quantities of refined AAV vectors remains a challenging, yet essential endeavor.
- Long-Term Safety: Although early studies suggest improved safety, long-term studies are required to ensure that these modifications do not introduce unforeseen complications.
- Regulatory Approvals: As with any emerging therapy, rigorous clinical trials and approvals from governing bodies are necessary. This regulatory process, while sometimes intimidating, is critical for patient safety.
There is also the socio-economic aspect of making such therapies widely available. It is not enough to prove that these therapies are effective; they must also be accessible and affordable. Co-development strategies and public–private partnerships are expected to play a critical role in achieving these goals.
Strategies for Managing Immune Responses in Gene Therapy
One of the significant hurdles in the use of viral vectors is the body’s immune response. A virus, even when engineered for therapeutic purposes, is still a foreign entity that can trigger defense mechanisms. Managing these nerve-racking immune responses is essential to ensure that the therapeutic gene is not neutralized before it reaches its target.
Scientists are employing various strategies to help steer through this challenge, including:
- Transient Immunosuppression: Briefly suppressing the immune system during vector administration to allow for successful gene transduction.
- Capsid Shielding: Modifying the surface of the AAV vector to camouflage it from immune cells.
- Targeted Delivery Mechanisms: Using techniques that guide the vector directly to its intended cells, reducing systemic exposure.
All these tactics are designed to lessen the likelihood of the vector being attacked by the immune system and to boost the overall efficiency of gene delivery. By getting around these intimidating hurdles, the road to safe and effective gene therapies becomes much clearer.
Weighing the Pros and Cons: A Balanced Perspective
As with any emerging technology, modified AAV vectors come with their own set of advantages and disadvantages. It is important to maintain a balanced view when assessing their potential for clinical use.
Advantages:
- Enhanced specificity for vascular endothelial cells, ensuring better targeted treatment.
- Increased transduction efficiency leading to more consistent gene expression.
- Potential for reduced dosage requirements, minimizing toxicity and side effects.
- Longer expression times which are particularly beneficial for chronic conditions.
Areas Requiring Further Research:
- Long-term safety and possible immune-mediated complications.
- Scalable production processes to meet clinical demands.
- Optimal administration protocols that balance efficacy with patient comfort.
- Cost-effectiveness analyses in comparison with other emerging gene therapy platforms.
This balanced view helps frame the conversation around gene therapy as one that is optimistic yet cautious. The promising results must be weighed against the hurdles that remain, and ongoing research is critical in addressing these unresolved issues.
Integrating Modified AAV Vectors into Future Therapeutic Strategies
The impressive progress in modifying AAV vectors for enhanced gene therapy is paving the way toward a new era of cardiovascular treatment. However, successful integration into standard clinical practice will require a convergence of scientific innovation, regulatory oversight, and collaborative partnerships between academia, industry, and healthcare providers.
Looking ahead, several strategies could help in the smooth adoption of these advanced therapies:
- Collaborative Consortia: Bringing together experts from various disciplines to share data and streamline research efforts.
- Incremental Clinical Trials: Conducting phased studies to validate the safety and efficacy of these vectors in human populations, starting with small patient cohorts and expanding as confidence grows.
- Patient-Centric Research: Involving patient advocacy groups early in the research process to ensure that the final therapies meet real-world needs.
- Robust Manufacturing Protocols: Establishing standardized manufacturing processes to maintain consistency and scalability in vector production.
These initiatives are essential for making sure that the benefits of modern gene therapy not only remain confined to the laboratory but also make their way into everyday clinical practice, positively impacting a vast number of patients suffering from cardiovascular conditions.
Technological Innovations Driving the Future of Gene Therapy
Further technological advancements are expected to drive progress in gene therapy beyond the current frontier. Researchers are continually exploring novel methods of vector modification and alternative delivery mechanisms. For instance, the ongoing development of CRISPR-based gene editing holds promise for even more precise genetic modifications that could further enhance the efficacy of therapies.
Additionally, advanced computational modeling is helping scientists predict how slight changes in viral design might affect performance, contributing to less trial and error in the laboratory. These computational tools can help scientists:
- Simulate interactions between modified vectors and cellular components.
- Identify potential weak points in vector design before going to in vivo trials.
- Optimize the balance between vector efficacy and safety in a controlled virtual environment.
The integration of these advanced tools not only speeds up the research timeline but also reduces the overwhelming cost associated with experimental gene therapy development. As a result, gene therapies are likely to become more accessible and refined in the near future, ushering in a new age of personalized and precision medicine.
Debating the Ethical and Social Implications
While the scientific merits of modified AAV vectors are clear, the path forward is not without its ethical and social considerations. As gene therapies continue to develop, it is important that the discourse remains balanced and that the potential social impacts are taken into consideration.
Among the ethical concerns are:
- Equity of Access: Ensuring that these advanced treatments are available to patients across different socio-economic backgrounds is key. No one should be left behind simply because of cost or location.
- Long-Term Effects: Even with promising early results, uncertainty remains regarding the long-term consequences of integrating modified viral vectors into the human genome.
- Informed Consent: Given the nerve-racking nature of gene therapies, it is absolutely critical that patients fully understand the benefits and potential risks before proceeding.
Constructive public debates alongside regulatory dialogues are essential to address these issues. Open communication between scientists, clinicians, and the wider community will be super important in ensuring that the pace of innovation is matched by an equally robust framework to support ethical practice.
Learning from Past Experiences and Future Directions
Gene therapy has experienced both significant breakthroughs and setbacks in the past. Learning from these experiences is a must-have part of progressing further. Historically, setbacks have taught the scientific community valuable lessons in terms of safety protocols, vector design improvements, and the overall patient experience.
Drawing on past lessons, future directions in vector engineering could focus on:
- Enhanced Screening Processes: Developing more refined preclinical screening methods to identify potential adverse reactions before starting clinical trials.
- Iterative Design Improvements: Utilizing a cycle of continuous feedback, where each clinical trial informs the next iteration of vector development.
- Adaptive Regulatory Frameworks: Creating flexible yet robust guidelines that can accommodate rapid technological advancements while protecting patient safety.
- Integrated Clinical Follow-Up: Establishing long-term follow-up protocols to monitor patients for decades after therapy administration, ensuring that any emerging complications are caught early.
This mindset of continuous improvement and careful oversight is crucial for dealing with the problematic, complicated pieces that naturally accompany revolutionary treatments. As researchers take a closer look at each new discovery, the eventual goal remains clear: to provide safe, effective, and widely accessible therapeutic options that address the underlying causes of disease rather than merely treating symptoms.
Building a Collaborative Ecosystem for Advanced Gene Therapy
The promise of modified AAV vectors can only be fully realized through a collaborative ecosystem where stakeholders across the healthcare spectrum work together. From academic institutions to biotech companies, from clinicians and regulatory bodies to patient advocacy groups, every entity plays a role in shaping the future of gene therapy.
Here are some key strategies for building such a collaborative environment:
- Interdisciplinary Research Networks: Foster partnerships among experts in virology, molecular biology, immunology, and clinical medicine to spark innovative ideas and accelerate the translation of research findings.
- Shared Data Platforms: Encourage open sharing of research data and clinical trial results to avoid duplication of effort and ensure that lessons learned are rapidly disseminated for the benefit of all.
- Joint Funding Initiatives: Combine resources from public and private sectors to support large-scale, multi-center studies that can generate high-quality evidence for the safety and efficacy of new therapies.
- Patient Engagement Programs: Include patients in the research process to gather real-world insights and ensure that emerging therapies truly address the needs of those they are designed to help.
By taking the wheel and steering through the twists and turns in the field of gene therapy, stakeholders can create an environment where innovation thrives and patient care is continuously improved. This collaborative spirit not only enhances the scientific community but also builds public trust in these pioneering treatments.
Concluding Thoughts on the Future of Modified AAV Gene Therapies
In summary, the development of modified AAV variants capable of efficiently delivering genes into human vascular endothelial cells is a monumental step forward in the realm of gene therapy. While there are still many confusing bits and nerve-racking challenges to overcome, the progress achieved thus far is undeniably inspiring.
Looking ahead, a few key points stand out as central to the future success of this technology:
- Enhanced vector specificity and efficiency promise to revolutionize treatments for cardiovascular and related conditions.
- Innovative methods to overcome immune reactions and ensure long-term safety are critical for broader clinical adoption.
- Collaborative, interdisciplinary efforts and patient-centered research will be the linchpins of future breakthroughs.
- Adapting regulatory frameworks and embracing ethical considerations will help maintain public trust and ensure equitable access to these therapies.
The journey from laboratory discovery to clinical application is full of tangled issues and complicated pieces, but the promise of truly transformative treatments makes every step worthwhile. With continued research and collaborative efforts across multiple disciplines, it is quite conceivable that modified AAV vectors will soon become a cornerstone of personalized, precision medicine for cardiovascular and other chronic diseases.
In an age where every patient’s well-being is of critical importance, these exciting advancements offer hope for a future where gene therapy is not just a tantalizing possibility but a standard, life-changing treatment. As the scientific community continues to figure a path through the tricky parts and hidden complexities of gene delivery, the ultimate reward will be a healthier, more resilient population made possible by innovative biotechnology that truly understands the fine shades of human biology.
As we steer through the evolving landscape of modern medicine, it is essential to remember that progress is often made one careful step at a time. Each new study, each small improvement in vector design, contributes to a larger mosaic of therapeutic advancements that promise to redefine how we treat diseases. By maintaining a balance between cautious optimism and rigorous validation, the field of gene therapy stands on the brink of transforming the way we approach some of the most challenging health issues of our time.
Ultimately, the story of modified AAV variants is one of perseverance, ingenuity, and collaboration. It reflects the tireless efforts of researchers committed to addressing the nerve-racking challenges and intricate problems of gene delivery. As these breakthroughs move from experimental stages to established clinical practice, they will undoubtedly redefine the future of treatment for cardiovascular disorders and beyond – a future where the complex bits of biology are managed with precision, care, and a relentless drive to improve human health.
In closing, the progress made in refining gene delivery methods using modified AAV vectors is both a cause for celebration and a reminder of the ongoing work needed to tackle the twisted issues associated with cutting-edge therapies. With every new development, the dream of curing previously intractable diseases becomes a little less daunting, and the promise of personalized, effective treatments shines brighter. The journey ahead may be full of challenges, but it is one that holds the potential to change lives and redefine modern medicine for generations to come.
Originally Post From https://www.geneonline.com/study-explores-modified-aav-variants-to-enhance-gene-delivery-in-human-vascular-endothelial-cells/
Read more about this topic at
Strategies for enhanced gene delivery to the central nervous …
Computationally guided AAV engineering for enhanced …