
Revolutionary Advances in CAR T-Cell Therapy for Multiple Myeloma
The recent findings from the CARTITUDE-1 trial have sparked significant attention among both clinicians and patients alike. In a patient group that had previously undergone multiple lines of treatment, a single infusion of a novel CAR T-cell therapy—ciltacabtagene autoleucel—yielded profoundly encouraging results. These findings suggest that for roughly one out of every three patients with heavily pretreated, relapsed or refractory multiple myeloma, long-term remission may be within reach without the need for subsequent or maintenance treatment.
This opinion editorial takes a closer look at the emerging evidence, the underlying mechanisms that could contribute to these outcomes, and the broader implications for future clinical practice and research in the field of oncology.
Understanding CAR T-Cell Infusion and Its Potential
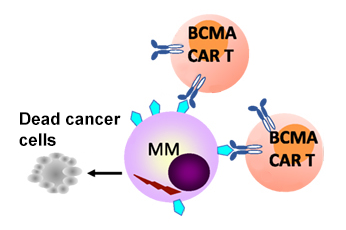
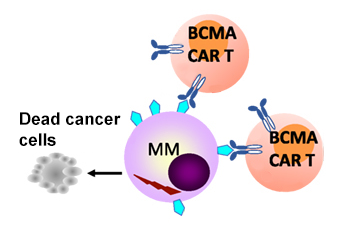
At the heart of this breakthrough is the process of autologous cellular immunotherapy. In simple terms, CAR T-cell therapy involves collecting a patient’s own T cells and genetically modifying them to target cancer cells more efficiently. Once reintroduced into the patient’s body, these reprogrammed cells seek out and destroy malignant cells with impressive precision.
The CARTITUDE-1 trial is particularly notable because it examined patients who had already been through several lines of treatment. These patients, who once faced an overwhelming disease burden and limited options, have shown improvements that not only meet but exceed traditional expectations. For many, the 5-year progression-free milestone achieved after just one infusion of treatment hints at the possibility of curative outcomes.
This kind of progress is inspiring for many reasons. It not only provides hope for patients who have experienced the nerve-racking twists and turns of a long, difficult treatment journey, but it also offers a glimpse into a future where treatments are less intrusive and more effective.
Clinical Trials Show Promising Long-Term Benefits
The details of the CARTITUDE-1 trial highlight the promising role of ciltacabtagene autoleucel. Out of the 97 patients initially enrolled—who had already exhibited progressive multiple myeloma and had undergone at least three prior lines of therapy—32 patients remained progression-free for at least 5 years after receiving a single infusion.
This remarkable statistic is more than just numbers on a page; it represents real hope for patients facing a condition once seen as nearly impossible to manage. Key observations from the trial include:
- A median progression-free survival (PFS) of approximately 34.9 months.
- A median overall survival (OS) that reached nearly 60.7 months.
- Approximately 45% of patients remained alive at the 5-year mark.
These outcomes are distinctly heartening when compared to real-world data where heavily pretreated patients with relapsed or refractory multiple myeloma had a median PFS of just 4.6 months and a median OS of 12.4 months. Such dramatic improvements underscore the transformative potential of CAR T-cell treatments in a patient population loaded with challenging issues.
Importantly, the trial’s long-term follow-up provided not only survival statistics but also important insights into sustained remission. In a subgroup of patients tested through annual measurable residual disease (MRD) assessments and PET/CT imaging, every one of the 12 patients maintained a complete metabolic response beyond the 5-year mark, strongly suggesting that the treatment might have cured or, at the very least, delivered an unprecedented durability of complete response.
Role of Measurable Residual Disease in Predicting Outcomes
A key aspect of the CARTITUDE-1 trial was its careful monitoring of measurable residual disease. Tracking MRD helps to clarify whether a patient maintains complete remission or if lingering traces of cancer might reemerge over time. In this study, achieving an MRD-negative status—even at very sensitive thresholds such as 10⁻⁵ or 10⁻⁶—was highly correlated with long-lasting remission and durable responses.
This detailed approach is critical for a couple of reasons:
- Indicator of Treatment Success: Patients who achieved MRD negativity were less likely to see cancer progression, indicating that the CAR T-cell therapy had effectively eliminated even the most hidden cancer cells.
- Guiding Future Therapies: Recognizing which patients reach these critical MRD benchmarks early on can help doctors figure a path for tailoring future treatment regimens or deciding on additional therapies to maximize long-term remission.
In the context of multiple myeloma, where disease monitoring is peppered with tricky parts and tangled issues, MRD testing offers clinicians a clear window into the subtle details of a patient’s treatment response, providing both reassurance and a benchmark for future therapies.
Comparing Real-World Data with Clinical Trials
Real-world evidence plays a crucial role in understanding how clinical trial results translate into everyday practice. For instance, the prospective registry study LocoMMotion provided insights into current standards of care in heavily pretreated patients with relapsed or refractory multiple myeloma. The differences between clinical trial data and real-world outcomes have always been a focal point of discussion during new treatment rollouts.
Some of the key contrasts include:
| Parameter | CAR T-cell Therapy (CARTITUDE-1 Trial) | Standard of Care (LocoMMotion Study) |
|---|---|---|
| Median Progression-Free Survival (PFS) | 34.9 months | 4.6 months |
| Median Overall Survival (OS) | 60.7 months | 12.4 months |
| 5-Year Survival Rate | Approximately 45% | Significantly lower |
This table underscores the stark contrast in outcomes between CAR T-cell therapy and current standard therapies. Such evidence suggests that for patients who have experienced the overwhelming burden of multiple prior therapies, CAR T-cell treatment emerges as a promising alternative that might significantly alter the trajectory of their care.
The Impact of Disease Burden and Immune Cell Characteristics
Diving into the study details, researchers observed that the burden of disease played a key role in determining long-term outcomes. When comparing patients who remained progression-free for 5-plus years to those who experienced disease progression earlier, clear differences emerged in both the bone marrow plasmacytosis levels and the concentration of soluble B-cell maturation antigen levels.
For instance:
- The progression-free group displayed a median bone marrow plasmacytosis of 5%, while the group with progressive disease had a median of 24%.
- The median soluble B-cell maturation antigen level was 36 µg/L in the progression-free group in contrast to 58.5 µg/L observed in patients who later experienced disease progression.
These observations suggest that starting with a lower burden of disease before CAR T-cell infusion may be a critical factor in achieving better long-term outcomes. Moreover, the cytokine milieu and the composition of effective immune cells within the CAR T-cell product prior to infusion appeared to correlate with patient outcome.
Specifically, patients who achieved long-lasting remission exhibited:
- A higher ratio of T cells to neutrophils.
- A greater proportion of naive T cells in the infusion product.
- An improved effector-to-target ratio at the time of peak CAR T-cell expansion post-infusion.
These subtle details add another layer of understanding to what makes CAR T-cell therapy effective for some patients. While more research is needed to tease out these little twists, these insights pave the way for further refinement of the therapy, ensuring that patient selection and treatment timing are optimized for the best possible outcomes.
Long-Term Safety: What Do the Findings Tell Us?
No discussion of a breakthrough treatment can be complete without addressing its safety profile. Given that CAR T-cell therapies are relatively new compared to traditional cancer treatments, evaluating long-term toxicity and related risks is crucial both for clinicians and those facing a cancer diagnosis.
In the CARTITUDE-1 trial, with an additional follow-up of about 28 months, the safety profile of ciltacabtagene autoleucel continued to align with previous observations. Notably, there were no new instances of conditions such as parkinsonism or cranial nerve palsy—which had been areas of concern following similar treatments—with only a few isolated adverse events reported. In particular:
- Two cases of secondary primary malignancies were identified—one lung adenocarcinoma and one squamous cell carcinoma of the anus.
- There were two new neurologic events, including a case of encephalopathy and a minor case of taste disorder, which were not directly linked to the treatment.
- Four new-onset grade 3 infections, such as appendicitis and upper respiratory tract infections, as well as one instance of urosepsis, were noted. These side effects were considered within the expected range and not directly attributed to the CAR T-cell therapy.
Sustained monitoring remains essential. The ongoing CARTinue postinfusion follow-up study will continue evaluating patients for up to 15 years, ensuring that any late-term toxicities are carefully cataloged. This long-term vigilance is critical, especially when a single treatment is positioned to replace years of continuous therapies.
Implications for Future Treatment Approaches
The success observed in the CARTITUDE-1 study shines a new light on the potential of immunotherapy, particularly CAR T-cell therapy, in treating relapsed or refractory multiple myeloma. As this treatment continues to be refined and investigated, several potential implications emerge for the future of cancer care.
Some key takeaways include:
- Earlier Intervention: The promising outcomes suggest that considering CAR T-cell therapy earlier in the treatment course might improve long-term remission rates even further. Upcoming trials such as CARTITUDE-4, -5, and -6 are already exploring these possibilities.
- Tailored Treatment Regimens: The differences noted in disease burden and immune cell composition indicate that individual patient factors could be leveraged to customize treatment plans. This personalized approach can help optimize which patients are most likely to benefit from the therapy.
- Better Risk Management: With a clearer understanding of the safety profile over an extended period, clinicians can better manage potential side effects and reassure patients. This management is especially important given the often nerve-racking twists and turns that come with traditional therapies.
These implications suggest that the role of CAR T-cell therapy could expand beyond the current patient populations into earlier stages of treatment. With additional research, this treatment may well evolve into a cornerstone therapy for multiple myeloma, reshaping clinical guidelines and patient expectations alike.
Challenges and Future Research Directions
Despite these exciting developments, it is crucial to recognize that many challenges remain. The journey from promising trial results to widespread clinical adoption is filled with tricky parts and confusing bits that require careful consideration.
Some of the main challenges include:
- Understanding the Full Mechanism: Although we know that CAR T-cell therapy works by reprogramming the immune system, the fine points of how these cells interact with the multiple myeloma environment are still being figured out. More research is needed to shine a light on these subtle parts.
- Optimizing Patient Selection: Identifying which patients will benefit most from the therapy involves analyzing hidden complexities such as disease burden and specific immune cell profiles. This step is critical for ensuring that patients are not left to face unnecessary risks.
- Managing Early Side Effects: While the long-term safety seems promising, the early side effects of CAR T-cell therapy can be overwhelming. Addressing issues like cytokine release syndrome in a timely and effective manner remains a key area of research.
- Economic Considerations: Beyond clinical efficacy, there is the equally important task of making these treatments accessible and affordable. The high cost of development and production of CAR T-cell therapies presents economic challenges that must be addressed for broader clinical implementation.
Researchers are actively working through these issues to ensure that the promising early results translate into everyday clinical success. Future studies will not only continue to monitor patient outcomes, but also strive to improve infusion protocols, enhance manufacturing processes, and develop supportive therapies that complement CAR T-cell treatment.
Detailed Analysis: The Importance of Early Disease Burden Management
One of the standout observations in the CARTITUDE-1 trial was the apparent benefit of having a lower burden of disease at the time of CAR T-cell infusion. As highlighted earlier, patients with a lower percentage of bone marrow plasmacytosis and reduced levels of soluble B-cell maturation antigen experienced more favorable outcomes, supporting the idea that early intervention is super important.
Breaking down this observation further:
- Bone Marrow Plasmacytosis: Patients whose bone marrow showed fewer plasma cells had a stronger chance of long-lasting remission. This suggests that once the tumor cell population reaches a critical level, even powerful treatments like CAR T-cell infusions may face a tougher battle.
- Soluble B-Cell Maturation Antigen: Lower levels of this antigen were associated with better responses. Monitoring these levels before treatment can offer clinicians a simple yet effective way to gauge potential treatment success.
For clinicians and researchers alike, these findings underscore the importance of early detection and treatment optimization. Rather than waiting until the disease becomes overwhelming, it might be beneficial to incorporate CAR T-cell therapy sooner in the treatment algorithm for multiple myeloma.
Moreover, these observations open up opportunities for integrating other supportive interventions before initiating CAR T-cell therapy, thereby reducing the inexplicable burden of disease and setting the stage for more effective treatment outcomes.
Immune Cell Profiles: Fine Points and Small Distinctions
An in-depth look at the immune cell profiles used in the study also provides insights into why some patients experience remarkable long-term benefits while others do not. The study found that the balance and types of immune cells present in the CAR T-cell product are of key importance. Two particularly significant observations included:
- Ratio of T Cells to Neutrophils: A higher ratio was correlated with better progression-free survival, suggesting that a robust presence of T cells might be critical in fighting off any residual disease.
- Proportion of Naive T Cells: Patients who had a greater proportion of naive T cells before infusion showed a strong resistance to disease recurrence. These fine shades of immune composition underline how even small distinctions can have a large impact on therapeutic success.
Understanding these subtle details not only provides clarity into the treatment’s effectiveness but also points to the potential for future enhancements. By tweaking the manufacturing process to favor a higher proportion of effective T cell subsets, it may be possible to further improve patient outcomes and reduce the risk of relapse.
Practical Considerations for Clinicians
For healthcare providers considering integrating CAR T-cell therapy into their treatment arsenal, several practical considerations arise. These include the timing of the infusion, the patient selection criteria, and the strategies for managing post-infusion monitoring and side effects.
Some key points to figure a path through include:
- Timing of Intervention: Early use of CAR T-cell therapy appears to be associated with better outcomes. Clinicians need to assess disease burden carefully to determine the optimal time for treatment.
- Selection Criteria: Ideal candidates for ciltacabtagene autoleucel treatment typically have an Eastern Cooperative Oncology Group performance status of 0 or 1, indicating that they are functionally well despite their cancer diagnosis.
- Post-Treatment Monitoring: The continued evaluation over 15 years through studies like CARTinue is crucial. This long-term follow-up not only helps in monitoring the durability of the response but also in catching any late-emerging side effects, ensuring that any emerging issues can be addressed promptly.
- Multidisciplinary Collaboration: Integrating CAR T-cell therapy requires collaboration among oncologists, immunologists, and specialized nursing staff. Such teamwork is essential to steer through the complexity of managing both the immediate and the longer-term responses.
By keeping these practical pointers in mind, clinicians can better manage the treatment journey, making sure that patients receive the maximum benefit from this promising therapy while minimizing potential risks.
Broader Implications for Cancer Treatment
The implications of the CARTITUDE-1 trial extend far beyond just the treatment of multiple myeloma. They hint at a broader transformation in the treatment of cancers traditionally seen as challenging to manage. The use of precision immunotherapy not only represents a key shift in therapeutic strategy but also offers hope for an era where cancers might be controlled with fewer infusions and less constant treatment.
Other areas where these findings could have an impact include:
- Personalized Medicine: The success seen in this study reinforces the idea that tailoring treatment to the individual’s unique disease profile is super important. By integrating genetic and immunologic profiling, future therapies may become even more effective for a variety of cancers.
- Reduced Treatment Burden: For patients who have long been subjected to continuous cycles of chemotherapy and radiation, a single, highly effective infusion represents a revolutionary shift. This approach could change the way we think about treatment schedules and long-term patient quality of life.
- Enhanced Research Collaboration: The promising results have already spurred additional studies (such as CARTITUDE-4, -5, and -6) that will further explore the boundaries of CAR T-cell therapy. This collaborative momentum is likely to encourage new partnerships between academic institutions, pharmaceutical companies, and regulatory bodies.
Ultimately, the ripple effects of this study could lead to a transformation not only in multiple myeloma management but also across a spectrum of hard-to-treat cancers. As our understanding grows, it will become increasingly clear that the seemingly overwhelming and scary challenges associated with advanced cancer are gradually being replaced by more refined, targeted, and successful treatment methods.
Conclusions and Looking Ahead
The CARTITUDE-1 trial stands as a compelling testament to the evolving landscape of cancer treatment. With its demonstration of long-term remission through a single CAR T-cell infusion, the study details an approach that is both innovative and hopeful to patients who have historically faced nerve-racking treatment journeys filled with complicated pieces and hidden complexities.
Key takeaways from the trial include:
- The potential for a single infusion to deliver long-lasting, possibly curative, outcomes in a population that has experienced multiple prior treatments.
- The importance of monitoring measurable residual disease as a predictor of long-term success.
- The significance of lower disease burden and favorable immune cell profiles in supporting durable responses.
- Reassuring long-term safety data that points to manageable side effects and rare occurrences of severe adverse events.
Looking ahead, while there remain several abundant challenges to work through, it is clear that these findings set a new benchmark in the management of multiple myeloma. More importantly, they open the door for further explorations into the use of CAR T-cell therapy across different cancer types and earlier in the disease trajectory.
For patients and clinicians, the path forward may involve combining this novel approach with other emerging treatments that collectively work to lighten the heavy toll of cancer. Ongoing and future clinical trials will be pivotal in refining the timing, patient selection, and overall management strategies that underpin these promising results.
As we continue to figure a path through the twists and turns of cancer treatment evolution, it is essential that both the research community and the wider healthcare field remain adaptable, collaborative, and open to innovative methods that challenge the old paradigms. The article from the CARTITUDE-1 study serves not only as a report of breakthrough results but also as an invitation to reimagine what is possible for patients battling multiple myeloma and other cancers.
In summary, the encouraging outcomes from long-term follow-up in the CARTITUDE-1 trial have the potential to redefine treatment protocols for relapsed or refractory multiple myeloma. With effective strategies that consider disease burden, immune cell composition, and timely intervention, future therapies may offer even greater hope. These transforming trends remind us that a once nerve-racking diagnosis does not have to define a patient’s journey, as modern therapies increasingly steer through the tangled issues of cancer care with ever-improving precision and care.
As we stand at this exciting crossroads in cancer care, it becomes clear that the integration of precision immunotherapies like CAR T-cell therapy could mark the beginning of a new era—one in which patients are given genuine hope for durable, long-term remission and even cure. It is an evolution fueled by scientific rigor, compassionate care, and the ongoing collaboration between researchers and clinicians worldwide.
Ultimately, this great leap forward encourages us to remain optimistic about the future. By taking a closer look at the promising evidence, unsnarling the tricky parts of treatment implementation, and embracing the subtle shifts in our understanding of cancer biology, we are better positioned to deliver super important, life-changing care to every patient in need.
Originally Post From https://ascopost.com/issues/july-25-2025/evidence-of-potential-cure-with-single-car-t-cell-infusion-for-some-patients-with-multiple-myeloma/
Read more about this topic at
CAR T-cell Therapy and Its Side Effects
Long-term outcomes following CAR T cell therapy